1. Calibration Scope
Before we start our calibration project and gather the needed resources, it is important to first consider what is our calibration scope. This is where we can determine our capability of how wide our services can reach and what instruments we can calibrate.
This is the reason why we exist as an internal calibration lab. We will mention here the parameters that we can calibrate or the calibration service that we can provide, the reference standards that we are using with their accuracy or uncertainty value, and the reference procedure or methods including reference guides.
When can we perform monitoring and measurement?
As an in-house calibration lab, we should also determine when can we execute our calibration job for us to control calibration activities. Below are some reasons why or when we can perform calibration:
Calibration is performed when a new instrument is installed or purchased
instruments that are mishandled during transfer (for example, dropped or fell down)
when instrument performance is questionable
calibration period is overdue
kept in an unstable environment for too long (exposed to vibrations or too high/low temperatures)
when a new setting or adjustment is encountered
when required by a 3rd party audit
when required by customers
when health and safety require monitoring
when required by regulatory bodies
If you know your scope and the reason why you perform calibration, you can easily determine and assess the suitability of the reference standards to be used for those instruments that needed calibration. It also means that you are familiar with your reference standards.
For example:
ABC lab exists to provide a traceable calibration of measuring and test equipment under Dimensional, Pressure, and Electrical that can directly or indirectly affect the quality of our products.
The table below details the scope of capabilities and reference standards used.
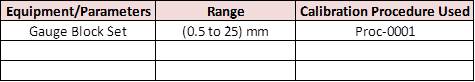
2. Recording System
I am sure before we start sending our instruments to an outside calibration lab, we already have a list of our measuring instruments.
If we have, this list is now our master list for our internal calibration laboratory (if you do not have one, you should create one, and take inventory of all the instruments for calibration).
It should include all the details of measuring instruments. This is now our starting point, we have now our inventory, we should determine where they are, who owns the instruments, and other details regarding the instruments like the calibration history file (instrument performance).
During this time, also take the time to put proper coding. This is now our monitoring tool. (in my past company, we have Calibration Management Software- the METCAL from Fluke which automates documentation and monitoring including the generation of calibration certificates). As a start-up, using an Excel sheet can already do the job.
Before, during, and after calibration, a recording is always a must. In calibration (whether internal calibration or external calibration), sometimes recording or documentation has more time to do than calibrating an instrument (so if you are a techy guy that hates writing or documenting a lot, this job will challenge you – just saying:-))
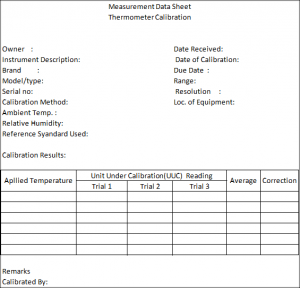
The Measurements Data Sheet (MDS) – Record Your Result!
You should have a form where your results and observations will be placed. I call this form a Measurement Data Sheet (MDS). Others call it a Measurement Data record, Calibration record, calibration worksheet, or company calibration form.
Why? Of course, to record and document your calibration execution. But another important reason is to have proper traceability regarding the performance, not just the execution but all the elements of the calibration system.
Just in case a problem arises regarding the instrument, we can easily trace back who perform the calibration and what procedure has been applied, and what data has been recorded etc.
MDS displays all the details regarding the UUC which includes: (Below is a sample Measurement Data Sheet (MDS))
Instrument Name
Instrument Description
Brand/Type/Model/Serial #
Equipment code
The environmental condition- Temperature and humidity
Date Received and Date Calibrated
Location of the Instruments
Range and Resolution
The Standard used during calibration
The Methods used in the calibration
The owner or department who use the Instrument
Results or data
The person who performs the calibration
Note that applicable Document Control and Records Control applies to all calibration procedures and forms used and other documents that are part of the calibration system.
Once the data sheet is completed, you can convert it to a Calibration Certificate with almost the same but some added details and more presentable with the title Calibration Certificate (to be more formal and adequate, it should be aligned with the requirements based on ISO/IEC 17025:2017, General requirements for the competence of testing and calibration laboratories).
To view the ISO 17025 calibration certificate requirements and tips to properly review and interpret the requirements of a calibration certificate, visit this link.
3. Calibration System (how we execute the internal calibration process)
The calibration system should detect and provide correction to any deficiencies related to calibration. A documented system that monitors and controls all measuring and test devices used in manufacturing a product, used in quality control or inspection operations to ensure conformance at given specifications or requirements.
Once we determine our scope, we will now specify what is the flow of our calibration process, how we can execute and control calibration jobs starting from collecting of the UUC to releasing of UUC with calibration certificates.
We will not just receive an instrument and then calibrate it immediately. We should have our first step before we proceed with calibration. Every step of a calibration process should be clear to us in order to have a smooth and documented execution.
In order to execute the internal laboratory calibration process properly and to have a clear view, below is a calibration process flow (or a plan) that I implement. You may change it depending on your needs but the steps or requirements should be maintained or more if necessary.
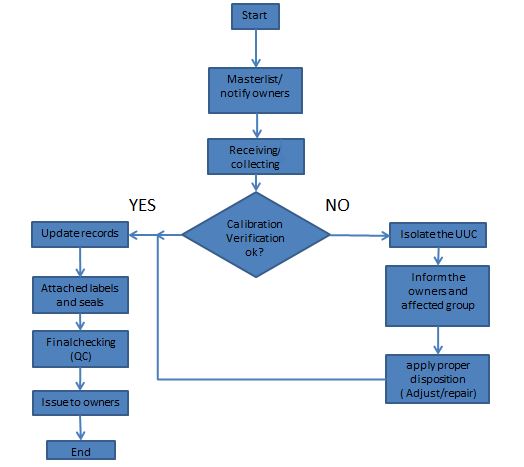
The calibration process presented above is the simplest, we did not consider putting other factors like what if we are not capable of calibrating a certain UUC, what if newly calibrated with an existing calibration certificate or totally damaged equipment?
These questions are usually tackled or solved by considering an additional process, but for simplicity, we will maintain it as is for the sake of this presentation.

















